
The second shell has six electrons ( 2 s 22 p 4) and needs two electrons to achieve octet.
Ionic charge periodic table download#
The electron configuration of O atom is 1 s 22 s 22 p 4. Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure 2). Don’t forget to download the HD image of the periodic table with charges from below. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. This electric charge generated on the ion is known as Ionic charge.

Up there when we talked about boron being negative, a negative ion, that is an anion. Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The general term when we're talking about a positive ion, we're talking about a cation. This is a platinum ion, a positive platinum ion.
Ionic charge periodic table plus#
How many electrons must O lose/gain to achieve octet? Write the formula of the resulting ion and its electron configuration. So you could write this as platinum with a plus four charge. ION CHARGE TRENDS Alkali Metals always have a +1 Ion Charge Alkaline Earth Metals all have a +2 Ion Charge Nitrogen and Phosphorus both have a -3 Ion Charge. Write the electron configuration of oxygen atom (Z=8). In macroscopic samples of sodium chloride, there are billions and billions of sodium and chloride ions, although there is always the same number of cations and anions. The number of electrons lost by the sodium atom (one) equals the number of electrons gained by the chlorine atom (one), so the compound is electrically neutral. Notice that there are no leftover electrons. The resulting combination is the compound sodium chloride. With two oppositely charged ions, there is an electrostatic attraction between them because opposite charges attract. First of all, let’s look at the group-wise ionic charge trends on the periodic table. The useful thing is that different elements have been grouped together on the periodic table so that its easy to see which ones share something in common.
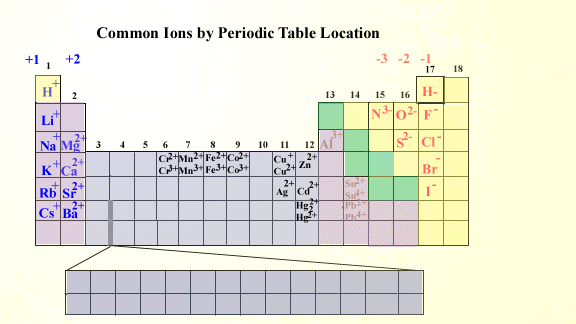
On the right, the chloride ion has 18 electrons and has a 1− charge. Method 1: By looking at the ionic charge trends on periodic table This is a very simple method to determine the ionic charge of the monatomic ions.

On the left, the chlorine atom has 17 electrons.


 0 kommentar(er)
0 kommentar(er)
